Abstract
Introduction: CD38 is expressed on the plasma membrane of lymphoid cells and in chronic lymphocytic leukemia (CLL) pts. increased CD38 expression is associated with a poor clinical outcome. While its role as a prognostic marker has been well established, the approval of daratumumab (Dara, first-in-class anti-CD38 mAb) offers a new role for CD38 as a potential therapeutic target in CLL. Matas-Cespedes et al have reported that Dara induces CLL cell death through antibody-dependent cellular cytotoxicity (ADCC), complement dependent cytotoxicity (CDC) and antibody-dependent cellular phagocytosis (ADCP). While CD38 is expressed on CLL cells, it is also expressed on T-cell subsets, such as Tregs. We hypothesized that in addition to its direct immune-mediated activity, Dara modulates the immune cellular environment; skewing T-cell populations from an immunosuppressive towards immune-reactive milieu, thus promoting immune reconstitution for enhanced anti-CLL response.
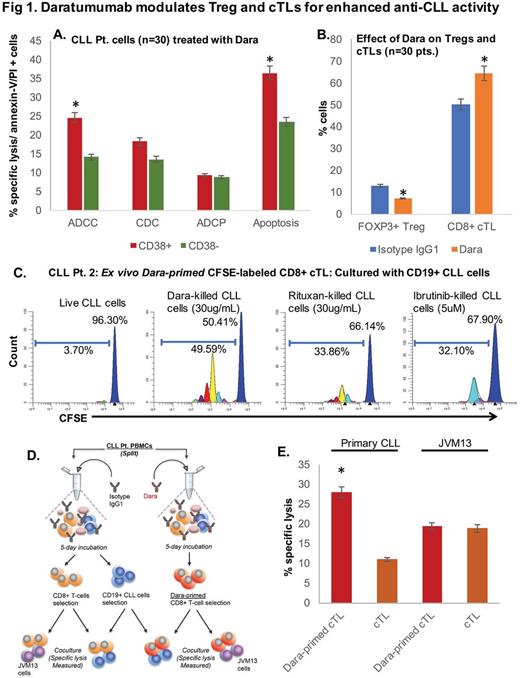
Methods: CD19+ cells were isolated from PBMCs of CLL pts. (n=30). Pts. with ≥30% CD38+ CLL cells were classified as CD38+ (n=22) and pts. with <30% CD38+ cells as CD38- (n=8). Dara-induced ADCC was assessed in Calcein AM labelled CLL cells cocultured with healthy donor PBMCs (E:T ratio, 50:1); CDC using 10% serum from a healthy donor and ADCP using human macrophages (E:T ratio, 2:1). Apoptosis was determined by annexin-V/PI staining followed by flow cytometry. Regulatory T-cells (Treg; CD3+CD4+CD25+CD127loFoxP3+) and cytotoxic effector T-cells (cTL, CD3+CD8+) were identified and isolated by FACS. Data are represented as mean±SEM.
Results: Dara (0.1ug/mL) induced significantly greater ADCC in CD38+ (24.63±3.46%) vs. CD38- cells (14.11±1.11%) (p<0.001), whereas CDC (18.22±2.41 vs. 13.58±0.95) and ADCP (9.37±0.77% vs. 8.79±0.92%) were marginally, but insignificantly, increased in CD38+ vs. CD38- CLL cases. We also observed significantly greater apoptosis in CD38+ (36.41±3.34%) vs. CD38- CLL cells (23.39±1.70%) (p<0.005) (Fig 1A). Immune repertoire analysis revealed lower CD3+ T-cell numbers in CLL pts. (11.45±3.25%) compared to those in healthy donor PBMCs (23.64±7.36%); but with a higher percentage of Tregs (10.71±2.47 in CLL pts. vs. 1.96±0.60 in healthy donors). To determine whether Dara could decrease CLL-Tregs, we treated CLL-PBMCs withDara (10µg/ml; 5 days) ex vivo, and noted significant decrease in Treg cells from 12.75±1.33 (Isotype control) to 7.17±0.91, (n=5, p<0.001). As downregulation in Tregs can consequentially lead to a rise in other T-cell subtypes, we observed increased effector cTLs: from 50.08±5.41 to 64.53±3.19 (p<0.02) (Fig 1B). Further analysis on CFSE-labeled CLL-cTLs (primed or unprimed with Dara), showed cTLs proliferated significantly more so when co-cultured with Dara-treated apoptotic CLL (51.75±1.16) cells vs. ibrutinib (31.44±0.36) or rituximab-treated apoptotic CLL cells (36.25±3.77) or live CLL cells (7.04±3.34) (Fig 1C, representative CLL pt.). To determine if Dara-primed cTLs had enhanced cytolytic aptitude towards autologous CLL cells, we cocultured primed cTLs with CLL cells. Compared to unprimed cTLs, specific lysis of target CLL cells was greater in the primed cTL-CLL coculture (12.61±1.75% vs. 28.27±0.82%, respectively). And this effect was noted to be patient-specific as cTLs cultured with JVM13 (CD38+) cells showed lower specific lysis (Fig 1D, E).
Conclusion: Here we demonstrate for the first time that Dara modulates CLL-Treg levels and increases effector cTL proliferation. Moreover, Dara-primed cTLs display heightened CLL-specific cytolytic activity than compared to Dara-naïve cTLs. Dara also induces direct apoptosis of CLL cells and exposure of Dara-killed CLL cells to cTLs increases cTL proliferation significantly more so than cTL exposure to CLL cells apoptosed using ibrutinib or rituximab. Altogether, our findings highlight the multiple mechanisms by which Dara (and potentially other anti-CD38 agents, currently being investigated by us) can induce CLL cell death and potentially revert dysfunctional cTL activity from that of a pro-CLL to an anti-CLL composure.
Parikh: AstraZeneca: Honoraria; Pharmacyclics: Research Funding; Pharmacyclics: Honoraria. Ding: Merck: Research Funding. Sher: LAM Therapeutics, Inc: Research Funding. Malavasi: Jaansen Pharmaceuticals: Research Funding. Ailawadhi: Amgen: Consultancy, Honoraria; Pharmacyclics: Research Funding; Takeda: Consultancy, Honoraria; Novartis: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal